Hi!Terribly Sorry for the delay
I'm Jordan Wong from TG02 and will be posting on a post on "Occult Blood Test"
OB TestOB Test refers to Occult Blood Test. Occult Blood Test is done usually to determine if there is any hidden (occult) blood in the stool/faeces. It helps us to find out whether if a particular person is suffering from any colorectal cancer or gastrointestinal bleeding (from mouth all the way to colon).
In order to perform the OB Test, stool samples from patients are collected as specimen and stored in an OB cassette, which will then be sent for processing using the Occult Blood Test Analyzer, Diana OC Sensor by Eiken.
1. Quality ControlAs usual, for all analyzers or machines that are used for running and processing samples, there is a need to run/perform Quality Control before the samples are sent for running. This is to ensure that the results/values the machine or analyzer is producing is precise and accurate. If not the results of samples may not be accurate, which may caused an analytical variation. Therefore Quality Control is an important procedure for all machines and analyzers in the laboratory.
Performing of Quality Control
• Prepare 4 Hitachi Cups for QC High and 4 Hitachi Cups for QC low
• Label the date (for example 24/07) and QC High/Low on the Hitachi Cups
• Take out the QC reagent from the fridge and let it cool to room temperature for 15 – 30 minutes
• Ensure and check that the Lot number of the QC reagent and the Lot number noted on the machine is the same before continuing
• Using the blue pipette, calibrate it to exactly 1000ul (1ml)
• Using the blue pipette, pipette exactly 1ml of deionised water into each QC reagent bottle
• To ensure well-mixed, Spin the QC reagent bottle for 15 – 30 minutes using the Nutating Mixer by GyroMini
• Pipette 200ul of QC reagent into each Hitachi cup (Total 4 cups)
• Remaining 200ul, pipette into each Hitachi cup drop by drop, ensuring that all 4 Hitachi cups has the same amount of well mixed QC reagent
• Place the Hitachi Cups for QC High and QC Low into the dark blue rack and send it for machine to run. [Dark Blue racks are meant for Quality Control]
• Wait for results to come out and ensure that the values produced for QC High and QC Low is with-in the reference range given by a standard/company
2. Preparation of SamplesFor OB Test in the laboratory, samples sent come in two types. Some come in OB cassettes while some come in stool bottles. As usual, samples that are sent were being signed, opened and registered. The samples will then be labelled, for OB cassettes; the label sticker must be labelled with the barcode facing the middle so that the sensor in the OB machine is able to read/scan the barcode into the system. OB cassettes has stool specimen in it, so the cassettes can be sent for processing right away.
However, some patients will send in Stool Bottle, where there might be other stool tests to be done other than Occult Blood (OB). Other stool tests may include Stool Culture and Stool FEME, thus a stool bottle containing the stool will be sent. Once we receive stool bottles from the admin department, we will go into LIS Edit Lab Request to check for the pending tests the patient enquired, if there is a pending test for Occult Blood, then there is a need to prepare OB cassette from the stool bottle.
As I said, in order to run Occult Blood for the ones with stool bottle, we will have to place the stool specimen from the bottle into the OB cassettes. This procedure will usually be performed in the safety cabinet at microbiology department.
• Label the OB cassette with the patient’s lab number
• Remove the cover of the OB cassette
• Place it into/onto the Stool Bottle, to retrieve some stool specimen
• Cover back the cover of the OB cassette
• Proceed to the Admin to print the correct label before the cassette is sent for processing
• During printing of label, enter the patient’s lab number, the code of the specimen, in this it will be 30 and click print
• Label the cassette properly, ensuring that the barcode is labelled on the middle of the OB cassette.
• OB cassette is ready for processing.
There are times where the stool cannot be scooped by the spoon of the stool bottle into the OB cassette, therefore we were taught to use ice cream sticks to retrieve some stool specimen and place it into the OB cassette.
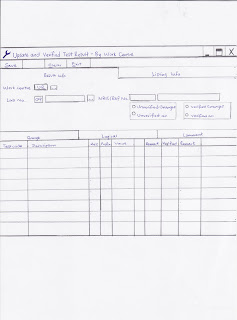
3. Merge Stool and OB cassettesThere are times where stool samples or OB cassettes do not come together on the day itself with other samples in the package. Thus these samples will arrive to the lab the next day, a few days later or even months later; we call these samples as merge samples. These merge samples come with a merge form. On the merge form itself, they will tick the type of sample. For example, merge urine is sent to the lab; the merge form will record and tick that urine is being sent. So upon receiving such merge samples, we will help merge the samples into the LIS system.
I. Ensure the samples received matches with the record on the merge form (A tick on the urine means only urine sample is sent)
II. In the LIS system, go to edit Lab Results, a screen will open; enter either the name or the IC number of the patient into the LIS.
III. A new window will open, now search for the recent package the patient has recently registered, record down the lab number of the patient.
IV. Now go to edit lab request, enter the lab number of the patient. Next, under cancel test and test info, ensure that the package has the test where the respective sample was sent. (Let’s say Urine Bottle was sent, ensure that the package includes urine test in the system)
V. If it correct, record the lab number on the merge form (0909090123) and also record down the day the sample was received.
VI. Under specimen information in edit lab request, enter the code for the specimen to register the samples into the system. (Urine Bottle = 23, Stool Bottle = 30, OB cassette = 31 & 32, Plain tube = 01, EDTA tube = 02 and Pap smear = 09)
VII. Ensuring what you merged was correct in the system, click saved. Next, go to print the label by entering the lab number and the respective code for the specimen.
VIII. Paste the label on the sample, highlight the lab number on the label and sent it to the respective department to run/process.
4. Running of SamplesAll OB cassettes and Stool Bottles will be sent to the Haematology Department and be placed into a yellow rack. In order to process/run the cassettes, we will place all OB cassettes into a light blue rack. Each light blue rack consists of 10 slots, which enable to hold 10 OB cassettes and a rack number is provided. [Light Blue racks are meant for processing/running of OB samples]
While slotting the OB cassettes into the test rack, we have to ensure that the barcode label of the OB cassette is in the middle. We also need to ensure that all papers or sticker must be removed from the OB such that it will not cause a hindrance when the OB is being processed. After ensuring that the OB cassettes are properly placed into the test rack, placed the rack onto the machine, aligning with the machine tray. Click set sample for the machine to run the OB cassettes.
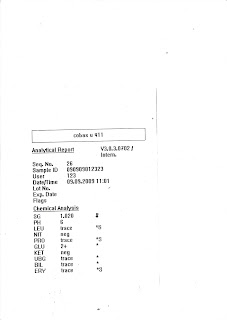
5. Verifying of ResultsOnce all the samples are processed/run, the light blue racks will be shifted to the left hand side of the machine, result slip will be printed out from the top. Then, a screen will appear saying analysis is done. The result slip consists of several information such as rack number and position, results of the OB sample, lab number of the patient and the number of OB cassette being processed. Upon completion of the analysis by the OB machine, we can then verify the results of the patient.
For verifying of OB results, we can verify in two ways, the first one is by Lab number and the other way is by Test Code. By verifying through Lab Number, enter the lab number of the patient, click under the logical column, under the Occult Blood row, and enter the results of the patient. 1 represent for Negative and 2 represent for Positive. In addition, for all positive cases, we will have to enter the value of the results in ng/mL under the memo corner. Lastly, click verify changes for whatever changes that was done and click save. After all those procedures as described, the patient’s results for OB were verified.
To verify using the Test Code method, we will open 2 LIS window, and enter two test codes, as Occult Blood has two test codes, 475700 and 475702. 475700 refer to Occult Blood while 475702 represent Immunological Faecal Occult Blood. Some samples are registered under 475700 test code while some are registered under the 476702 test code. Thus we open both to ensure that all registered OB samples appear in the system so as to verify them. However, using this method will hang the LIS system in the whole lab, therefore it is not often used unless necessary like when checking pending only. Verifying is usually done through lab number. After opening both test codes, once again enter 1 which represent for Negative and 2 which represent for Positive. In addition, for all positive cases, we will have to enter the value of the results in ng/mL under the memo corner. Clicks verify changes for whatever changes that was done and click save.
6. Closing of OB machineOccult Blood Machine only run OB cassettes from morning till afternoon about 4pm only. After which, the machine will be closed. Before closing OB machine, we will usually check OB pending first where it serves to ensure/ to make sure that all OB cassettes that were registered are processed and verified or if it wasn’t processed then must ensure that the OB cassette is present for processing the following day. After checking OB pending, if there are no problems, then the OB machine can be closed.
• From the analysis screen, Press/Click close
• Wait till screen appears as Main Screen
• Press/Click Maintenance
• Press/Click Close Mode
• On the next screen, ensure that that it is Yes No Yes.
7. Checking Occult Blood Pending
Occult Blood Pending is done three times daily. Once in the morning, after running a batch of samples that was left during the previous day after the OB machine was closed. And another once more, in the afternoon, before the machine is closed at 4pm. Lastly, one more time of pending after all OB cassettes / Stool samples are merged and registered.
To check Occult Blood Pending, 2 LIS windows must be logged in. Then, open the two test codes for OB, 475700 (Occult Blood) and 475702 (Immunological Faecal Occult Blood). Wait till both test codes are completely opened. Then, look at both the screens; ensure that there are no samples listed in the screen. If there is, it is either not verified yet or the samples are just registered. Therefore, make sure that all OB results are verified. If samples are not process yet, make sure there are available for processing the following day.
As stated, after all OB cassettes / Stool samples are merged and registered. Open the OB pending for the third time. Using Microsoft Excel, print the OB pending (475700 and 475702) and ensure that the list of samples found in the pending matches with the number and the correct OB cassette(s) that were registered.

Transferring of Stool Specimen from Stool Bottle into OB cassette

Transferring of Stool Specimen from Bottle to OB cassette

This is a Stool Bottle : Used for Preparation of OB cassette specimen and other Stool Tests

These are all the require solutiones for Occult Blood Machine.
They are mainly Purified Water, Wash Solution and Drain (Waste)

This is our lab's Occult Blood Machine

10 OB cassettes slotting into 10 slots on the Light Blue Racks

This is a OB cassette

This is the Light Blue Racks for Patient's OB cassettes to be processed

Placing Stool from Stool Bottle into OB cassette

This is the Dark Blue Racks for running Quality Control in the OB machine before processing patients samples.

This is the Orange Racks used for running samples that requires Dilution in the OB machine.
Do you have any questions, do post it on our blog
This is all i have to share for today!
2 MORE DAYS!
Enjoy your final moments!
With Regards
Jordan Wong Wei Jie
TG02 0703992H
Group 9