ELISA, also known as enzyme-linked immunosorbent assay, is used in my laboratory to test for the concentration of cyclic GMP (guanosine monophosphate) or cyclic AMP (adenosine monophosphate)in cultured corpus cavernosum cells.
Cyclic GMP and cyclic AMP are secondary messengers that are involved in the relaxation of the penis. Therefore, an increase in these levels in the corpus cavernosum smooth muscle cells when treated with the test drug would prove that the drugs actually have relaxation effect on the penis.
In my lab, we use commercially purchased competitive ELISA kits to perform these assays. Before performing the assays, we have to culture the smooth muscle cells to passages 2 - 5. The lower passages are encouraged to be used so that the cells remain healthy.
When we get confluent flasks of smooth muscle cells, we seed them to 6 well plates to be grown to confluence. This usually takes a day for the cells to reach confluence in a 6 well when about 4.0x105 cells are seeded per well.
The experiment is continued on the next day, when the wells are confluent. The spent DMEM is removed from the wells and new FBS-free DMEM is added to the wells, followed by addition of IBMX (isobutylmethylxanthine), which is a non-specific inhibitor of phosphodiesterase (PDE), and the test drugs. We add IBMX because we do not want the PDE to break down any cyclic AMP and GMP so that we are able to detect them at the end of the experiment. The test drugs that I use are testosterone and a plant extract that is supposedly able to increase the cyclic AMP and GMP levels. Control wells have no drugs added to them.
The wells would be incubated and the supernatant would be removed from the wells. Hydrochloric acid is added to lyse the cells followed by physical lysing with a scraper. The supernatant would then be spun so that the supernatant would be separated from the cell pellet. Supernatant would be collected and diluted in different concentrations and then acetylated. The acetylated samples, together with antiserum (specific to cAMP/cGMP) and tracer (which is acetylcholinesterase linked to cAMP/cGMP) are then loaded into the 96-well plate that is coated with mouse monoclonal anti-rabbit IgG and incubated for 18 hrs.
After 18 hrs, the plates are washed and the substrate to acetylcholinesterase is added. The plate is then incubated for 30-90 minutes to allow enzymatic reaction to take place where a yellow colour would be produced. The plate is then read with a spectrophotometer.
The intensity of the colour would be proportionate to the amount of tracer that binds to the well, which would be inversely proportionate to the amount of free cAMP/cGMP that is found in the sample. Therefore, the higher the reading of the plate, the lower the amount of cAMP/cGMP is found in the sample. Therefore, to show that my drugs are working, I would have to obtain cAMP/cGMP levels that are higher than the control.
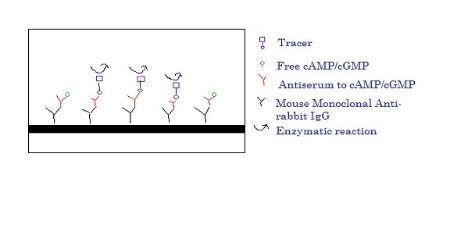
Sorry for my ugly drawing. But hope that explains to you all about the assay that I am conducting. Feel free to ask questions: )
Love,
Renee
TG02
0703634F
Yo Reneeeeeeeeeeeeeeeeee =D
ReplyDeleteHey, so basically, the concept is that the tracer competes with the free cAMP/cGMP in acetylated samples for the binding sites of the anti-cAMP/cGMP, rite?
And wanna clarify with u about the procedure ^^ so u add antiserum 1st, followed by mixture of acetylated samples and tracer or u mix all of them together, then add into the wells?
Ya, that's all, lolz
Take care
Vo Thu Hong Anh [Jess]
0705346H
YO JESS DEAR!
ReplyDeleteHAHA! yupyups, u are right! the free cAMP/cGMP will compete with the tracer for the binding sites: )
The procedure is like that: We add the sample first, then add the tracer, followed by the antiserum: ) Its added consecutively, one after another. Its not mixed together, its added one by one: )
Love you Jess, see you soon!
Renee
TG02
0703634F
okayyyyyyy, got it, ahhaha, thankz Renee,
ReplyDeletemisss uuuuuuu also! =D
Vo Thu Hong Anh [Jess]
0705364H
Hey renee,
ReplyDeleteWhat do you mean by passages 2-5?
Felicia
0703345I
Hello Felicia,
ReplyDeleteThe definition of passage means the number of times the cells are subcultured. Therefore, it means that when the cells are confluent, i would trypsinize the cells and then split them into new flasks to let them grow again. When i do that, the passage number would change from 1 to 2. So i would be able to split the cells 5 times and use these cells for my culture because they are healthier.
Renee
TG02
0703634F
Hi renee!!!!!!!
ReplyDeletejust want tio ask... what do you mean by acetylated??
Thanks:)
Gwendolynn
TG02
0703953J
Hey gwen!
ReplyDeleteacetylation means addition of potassium hydroxide and acetic anhydride to the sample to detect low concentrations of cyclic AMP/GMP in the sample.
Renee
TG02
0703634F